Latent heat of fusion when changing between solid or liquid state for common materials like aluminum ammonia glycerin water and more. Register your Fusion and access the Hotronix Online Portal to manage users generate usage reports and troubleshoot issues.
How To Build A Heat Of Fusion Graph Quora

Values Of The Latent Heat Of Fusion Dfush And Melting Temperature Download Scientific Diagram
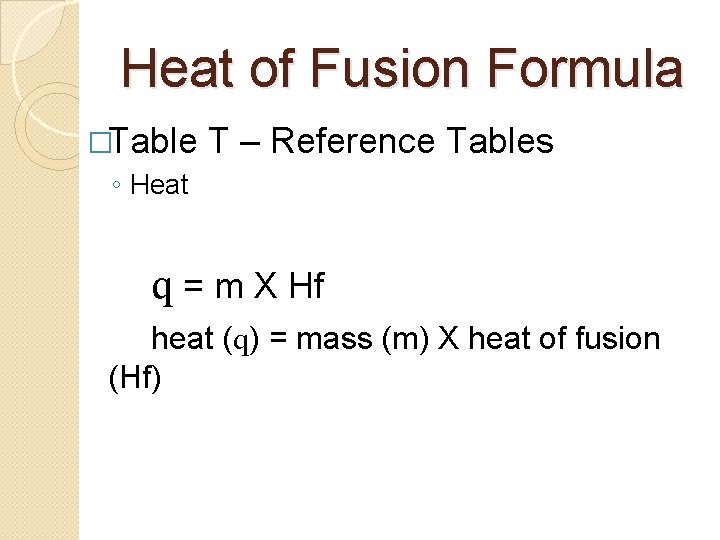
Heat Of Fusion Hf Energy Needed To Melt
The distinction between heat and temperature is subtle but very important.
Heat of fusion. Plus a brand-new controller with a larger higher resolution screen makes it easier than ever to. This heavy-duty version of the FUSION Heat Press is designed for high volume work powered by an air compressor. L latent heat of fusion kJkg L mol latent heat of fusion kJmol A atomic weight gmol Note that atomic mass and atomic weight are two different things.
A substance condensing or vaporizing at a specified temperature and pressure. Q ΔH fus massmolar mass The meanings are as follows. Its called the heat of fusion because when you fuse something together you make it solid.
A new approach to testing the capacity for spacecraft heat shields to withstand orbital re-entry makes use of the extreme temperatures found at the centre of nascent fusion reactors. This practice does not purport to address all possible heat fusion joining procedures or to preclude the use of qualified procedures developed by other parties that have been proved to produce reliable heat fusion joints. The latent heat of fusion or melting of a solid is the quantity of heat in joules required to transform a solid at its melting point to a liquid without any variation in temperature.
Other suitable heat fusion joining procedures are available from various sources including pipe and fitting manufacturers. Since its value is generally much higher than specific heat it allows you to keep a beverage cold for much longer by adding ice than simply having a cold liquid to begin withIts also why frozen meat takes a long time to thaw but once its thawed it heats up. Your source for all Fusion products.
Spacecraft have long used heat shields for protection during entry into planetary atmospheres. Just two different words for the same thing depending on what direction you going. Similarly you have the heat of vaporization.
Functioning as either a swinger or draw press the 16 x 20 Fusion IQ offers a heat-free workspace touch screen settings and live digital time temperature and pressure readouts. With two heat printing stations the Dual Air Fusion IQ heat press increases efficiency by allowing threading and layout on one station while the other is being pressed. Is defined through the formula Q.
Exothermic phase changes release heat to the environment. Its perfect for high-volume operations and for DTG printing set one station for pre-treatment the other for post-curing. Atomic weight is the average mass of all the isotopes of an.
It has only one value for water because water freezes at one value 0 C and it is 7971 calg or the rounded number 80 calg. So heat of fusion. Heat of fusion is the amount of heat energy required to change the state of matter of a substance from a solid to a liquidIts also known as enthalpy of fusion.
The amount of heat required to convert a unit mass of the solid into liquid without change in temperature. Latent heat of fusion is the heat consumed or discharged when matter melts changing stage from solid to fluid-structure at a consistent temperature. Latent heat of fusion is calculated as.
Latent heat can be understood as energy in hidden form which is supplied or extracted to change the state of a substance without changing its temperature. Now with Fusion IQ Controller The worlds most advanced heat press just got smarter. Examples are latent heat of fusion and latent heat of vaporization involved in phase changes ie.
They are warming processes The specific latent heat L of a material is a measure of the heat energy Q per mass m released or absorbed during a phase change. Experiment 4-Heat of Fusion and Melting Ice Experiment In this lab the heat of fusion for water will be determined by monitoring the temperature changes while a known mass of ice melts in a cup of water. It is also a latent heat and is sometimes called the latent heat of fusion.
The heat of fusion for water at 0 C is approximately 334 joules 797 calories per gram and the heat of vaporization at 100 C is about 2230 joules 533 calories per gram. Plus a brand-new controller with a larger higher resolution screen makes it easier than ever to modify settings and save recipes. In other words heat is energy while temperature is a measure of energy.
High-tech power with superior ease of use. The worlds most advanced heat press just got smarter. Molar heat values can be looked up in reference books.
Atomic mass is the mass of an individual element. The molar heat of fusion equation looks like this. 1 q is the total amount of heat involved 2 ΔH fus is the symbol for the molar heat of fusion.
The experimentally determined value for heat of fusion. Its units are usually Joules per gram Jg or calories per gram calg. The whole magnetic confinement fusion approach basically boils down to holding a plasma together with magnetic fields and then getting it as.
Education corporate office government healthcare hospitality resident living retail. The enthalpy of fusion is a latent heat in light of the fact that during softening the heat energy expected to change the substance from solid to fluid at air pressure is the latent heat of fusion as the temperature stays steady during. The heat of fusion is the quantity of heat necessary to change 1 g of a solid to a liquid with no temperature change Weast 1964 p.
Easily threadable with convenient EZ-On platens and hands-free auto-swing it sets a new standard for heat presses. The enthalpy of fusion of a substance also known as latent heat of fusion is the change in its enthalpy resulting from providing energy typically heat to a specific quantity of the substance to change its state from a solid to a liquid at constant pressureFor example when melting 1 kg of ice at 0 C under a wide range of pressures 33355 kJ of energy is absorbed with no temperature. Heat refers to the transfer of energy between systems or bodies whereas temperature is determined by the energy contained within a singular system or body.
The amount of heat gained by a solid object to convert it into a liquid without any further increase in the temperature is known as latent heat of fusionThe content of latent heat is complex in the case of sea ice because it is possible for sea ice and brine to exist together at any temperature and melt at a temperature other than 0 o C when bathed in a concentrated salt solution just like. L 1000 L mol A 1 where. Register your FUSION and access the Hotronix Online Portal to manage users generate usage reports and troubleshoot issues.
This value is a constant for a given substance. The important thing is the number 333. Because the heat of vaporization is so large steam carries a great deal of thermal energy that is released when it condenses making water an excellent working fluid for heat engines.
So it could also be considered the heat of melting. The latent heat of fusion is the heat required for an object to go from the solid state to the liquid state or vice versa. The latent heat of fusion of ice is 334 x 10⁵ joules per kilograms or 334 x 10⁵JKg.
Pneumatic regulator which auto-adjusts pressure. The latent heat of fusion of a substance is. Plus with industry-exclusive Threadability you can position a garment once rotate and decorate any area.
Heat Of Fusion Of Ethanol From Dortmund Data Bank
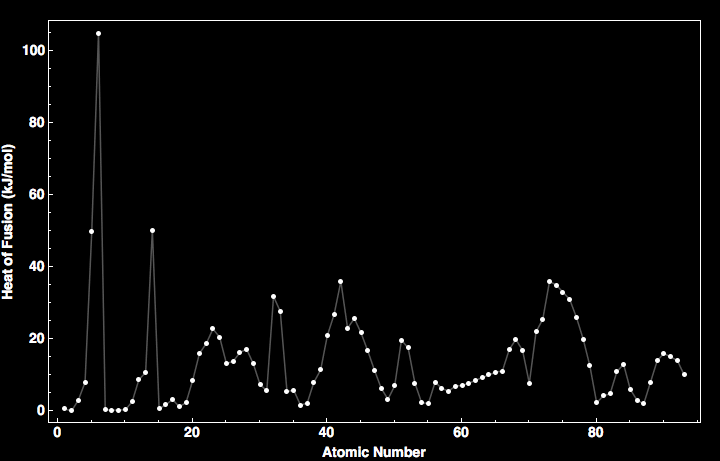
Heat Of Fusion For All The Elements In The Periodic Table
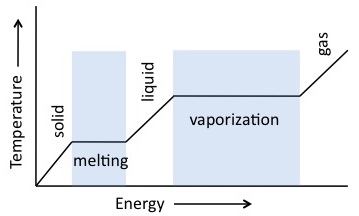
Heat Of Fusion Definition Equation Examples Video Lesson Transcript Study Com
Heat Of Fusion

Latent Heat Of Fusion And Vaporization Specific Heat Capacity Calorimetry Physics Youtube
Specific Latent Heat Of Fusion Of Ice International Baccalaureate Physics Marked By Teachers Com

Difference Between Latent Heat Of Fusion And Vaporization Compare The Difference Between Similar Terms

1 Latent Heat Of Fusion And Vaporization For Certain Substances Download Table

