Satellite measurements of infrared spectra over the past 40 years observe less energy escaping to space at the wavelengths associated with CO2. 42 14 3.

Solution Determine The Empirical Formula Chemistry

Determining Empirical Formula From Combustion Analysis Ppt Download
If A Hydrocarbon On A Complete Combustion With Oxygen Gives 0 54 G Water And 0 88 G Co2 Then What Will Be The Formula Of Hydrocarbon Quora
1 liter of the hydrocarbon weighs 126 g at NTP.
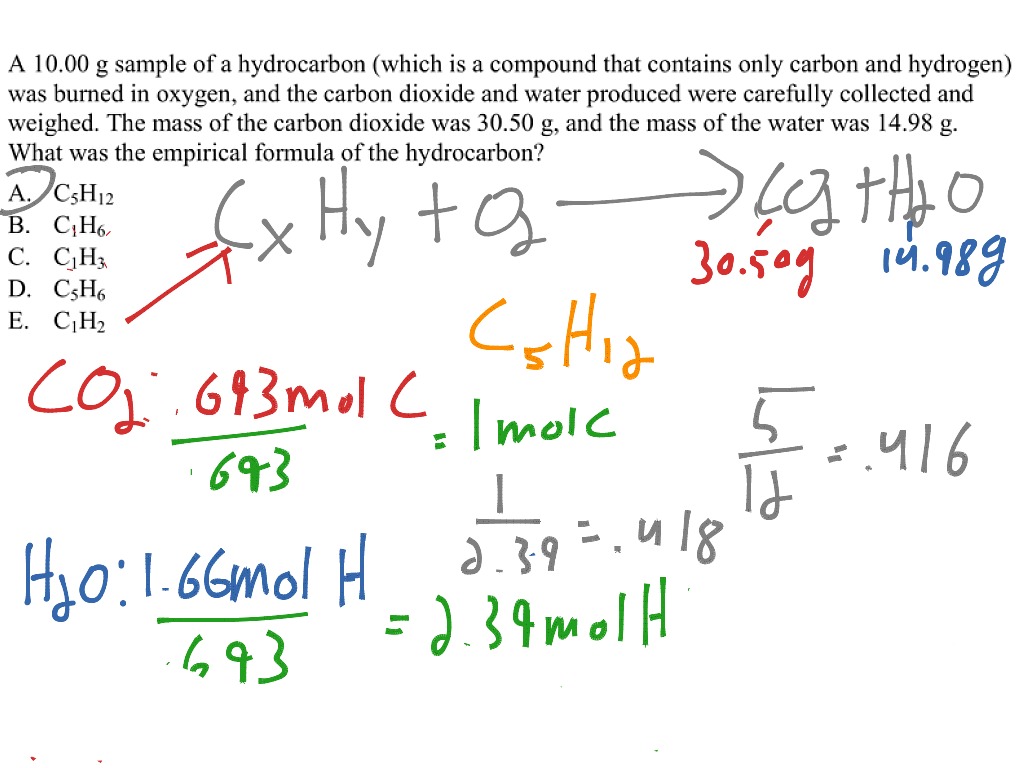
Hydrocarbon empirical formula. Which of the following could be the molecular formula of the compound. The empirical formula was determined in the Combustion Analysis tutorial to be CH. Answers appear after the final question.
What is the chemical formula of a diamond. Empirical formulas will be derived from the molecular formulas. The a suffix indicates that the isomer is unbalanced by one atom giving 1112-Tetrafluoroethane.
What is the percent C by mass for the molecule that has the ketone functional group. We know this because the transfer of a mole of hydrocarbon from pure hydrocarbon to dilute aqueous solution has an enthalpy of around zero. An empirical formula of a substance is found using the masses and relative atomic masses of the elements it contains.
Draw the geometric isomer of any that can. However the bases for the empirical equations are contained in other sections of this standard or the appropriate reference document. View Answer A 000215-gram sample of polystyrene a polymer composed of carbon and hydrogen produced 000726 grams of CO2 and 000148 grams of.
Surface measurements find more downward infrared radiation warming the planets surface. Given That Its Vapour Density Is 29Find Its Molecular Formula. Which of these can have a geometric isomer.
It is the water. A Gaseous Hydrocarbon Contains 8276 Of Carbon. The empirical equations for the coefficient of discharge and expansion factor are pre sented.
A hydrocarbon is found to contain 48 g of carbon and 10 g of hydrogen. How do empirical formulas and molecular formulas differ. C 12 H 1.
Methane is the simplest hydrocarbon possible. What is the empirical formula for the hydrocarbon. For the proper use of this standard a.
The shear viscosity or viscosity in short of a fluid is a material property that describes the friction between internal neighboring fluid surfaces or sheets flowing with different fluid velocities. A gaseous hydrocarbon contains 857 carbon and 143 hydrogen. The products were 388 mg of CO 2 and 317 mg of water.
This term empirical formula weight abbreviation EFW IS NOT a standard chemical term so be alert to how others describe it. Because of this terpenes usually have 5n carbon atoms n is an integer and are subdivided as follows. Instead the number and structural organization of carbons is a definitive characteristic.
10 and is the smallest hydrocarbon alkane that has isomers. The empirical formula of the hydrocarbon will thus be C_1H_2 implies CH_2 Answer link. Metric or SI Le Système International dUnités prefix are based on powers of tenThey are modifiers on the root word that tell us the unit of measure.
Which of the molecules below are polar. The molecular formula for these compounds is. Terpenes may be considered to be made up of isoprene more accurately isopentane units an empirical feature known as the isoprene rule.
For example R-134a has 2 carbon atoms 2 hydrogen atoms and 4 fluorine atoms an empirical formula of tetrafluoroethane. Get the answer to this question and. The company developed a hydrocarbon mix of isopentane and isobutane.
Which of the molecules below has the empirical formula C 3H 6O. Then the empirical formula of the oxide is A. 1 Determine the grams of carbon in 440 g CO 2 and the grams of hydrogen in 270 g.
This is mainly due to the self-bonding or catenation of carbon that prevents the complete saturation of the hydrocarbon by the formation of double or triple bonds. The formula for an alkane is CnH2n2. This friction is the effect of linear momentum exchange caused by molecules with sufficient energy to move or to jump between these fluid sheets due to fluctuations in their motion.
This means that the number of hydrogen atoms equals twice the number of carbon atoms plus two. The mass of the atoms in the empirical formula is 14. A hydrocarbon gas with an empirical formula CH_2 has a density of 188 grams per liter at 0C and 100 atmosphere.
Menthol the substance we can smell in mentholated cough drops is composed of C H and O. Empirical formula mass EFM 3 times 12 4 times 1. Determine the molecular formula of the hydrocarbon.
Identify the functional groups in each of these molecules. An enhanced greenhouse effect from CO2 has been confirmed by multiple lines of empirical evidence. The four carbon atoms of Butane can arrange themselves in two different manners resulting in a straight-chain structure and branched-chain structure.
Complete combustion of a sample of a hydrocarbon in excess oxygen produces equimolar quantities of carbon dioxide and water. The molecular formula of a compound is a representation of the number and type of elements present in one molecular unit of the compound. What is the empirical formula of this compound.
Empirical formulas are usually obtained from the analysis of experimental data. What is the. The empirical formula for glucose is CH2O.
The empirical formula of hydrocarbons is also different from each other. This 10-question practice test deals with finding the molecular formula of chemical compounds. A periodic table will be required to complete this test.
In order to correctly convert one metric unit to another you will need to determine which of two prefixes represents a bigger amount and then determine the exponential distance between them. A 150 g sample of hydrocarbon undergoes complete combustion to produce 440 g of CO 2 and 270 g of H 2 O. From that we can determine the empirical formula weight to be 13 one carbon plus one hydrogen.
Water drives non-polar substances out of the aqueous phase. A hydrocarbon engages in favorable molecular interactions with water in aqueous solution. So you need to multiply the numbers in the.
So why dont oil and water mix. What is a possible formula for the hydrocarbon. What is the empirical formula of the hydrocarbon.
For example the empirical formula of a hydrocarbon is CH 2 and its M r is 42. Find the empirical formula for the hydrocarbon. A 141 mg sample of a hydrocarbon was burned in air.
A hydrocarbon fuel is fully combusted with 18214 g of oxygen to yield 23118 g of carbon dioxide and 4729 g of water. How do you find molecular formula of a compound. Structural formula-The structural formula of a chemical compound provides insight into the arrangement of the atoms within the molecule.

Chemistry 101 Determining Molecular Formula Using Combustion Analysis Youtube

Empirical And Molecular Formulas

4 3 Empirical And Molecular Formulas Problems Chemistry Libretexts

Determining The Empirical Formula Of A Hydrocarbon Science Chemistry Elements Atoms Molecules Chemical Reactions Stoichiometry Showme
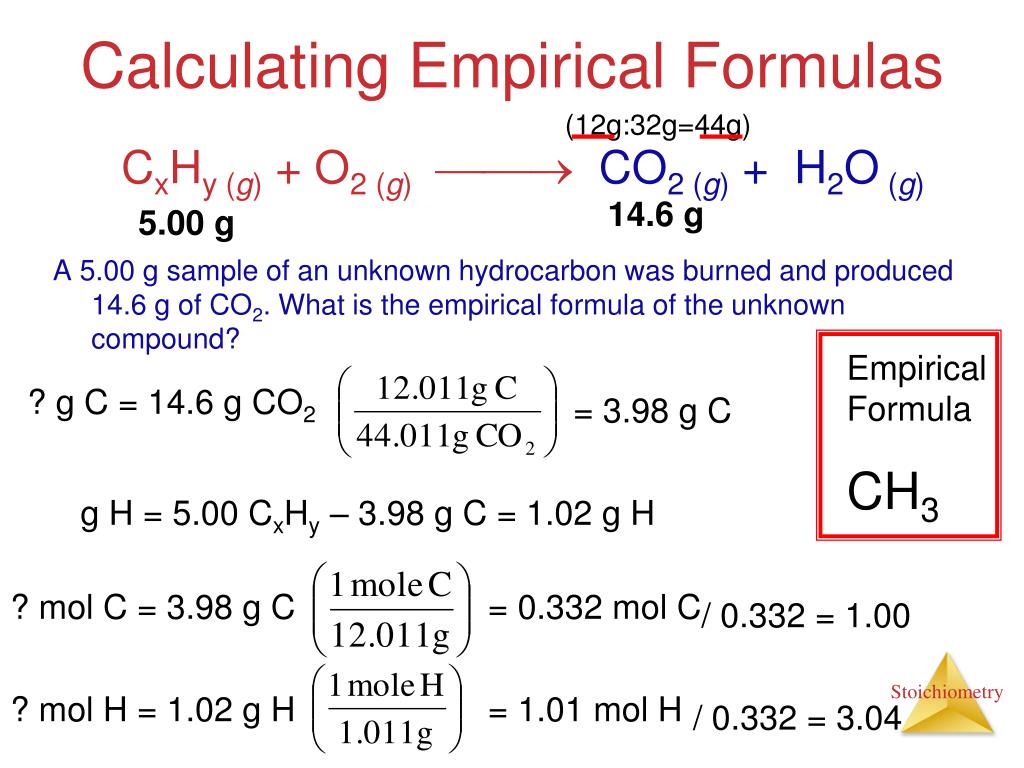
Ppt Unit 3 Stoichiometry Calculations With Chemical Formulas And Equations Powerpoint Presentation Id 3623549
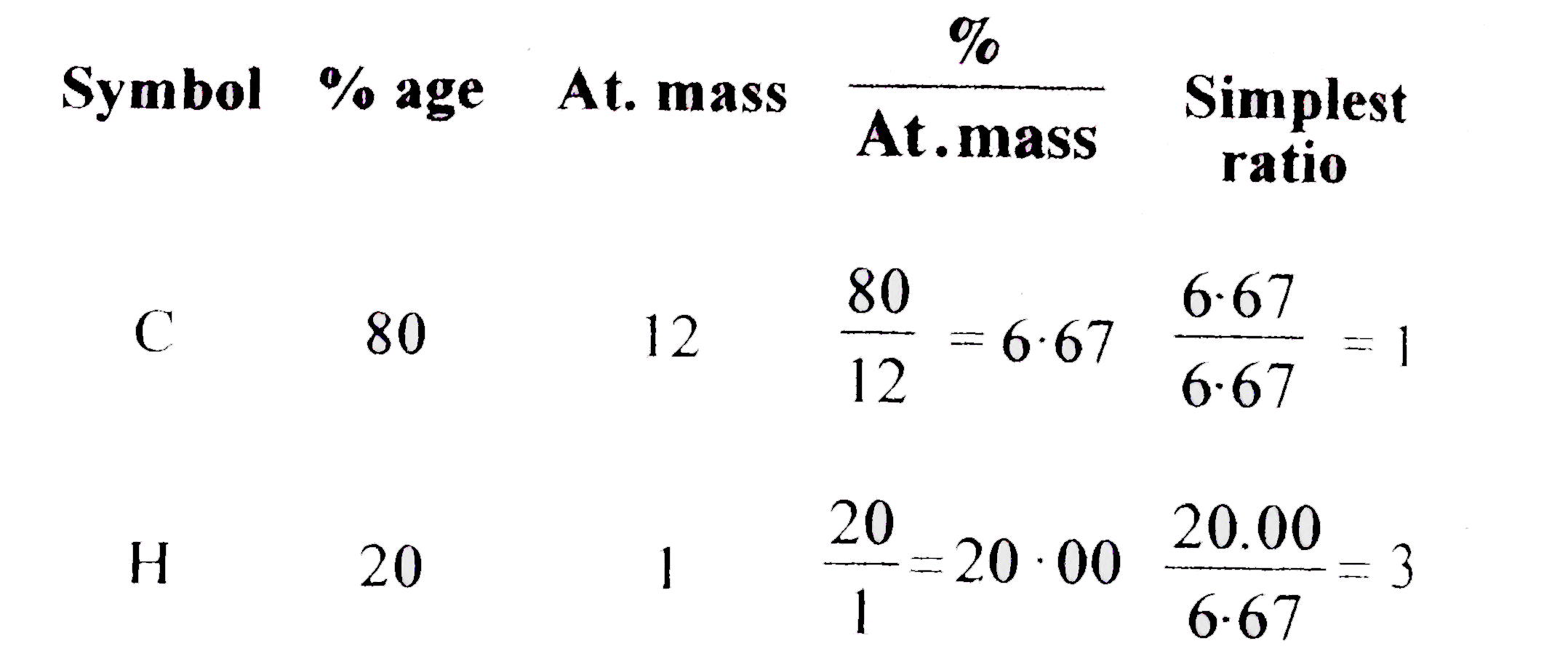
Emprical Formula Of A Hydrocarbon Containing 80 Corbon And 20 Hydrogen Is

Answered A Sample Of Hydrocarbon On Complete Bartleby
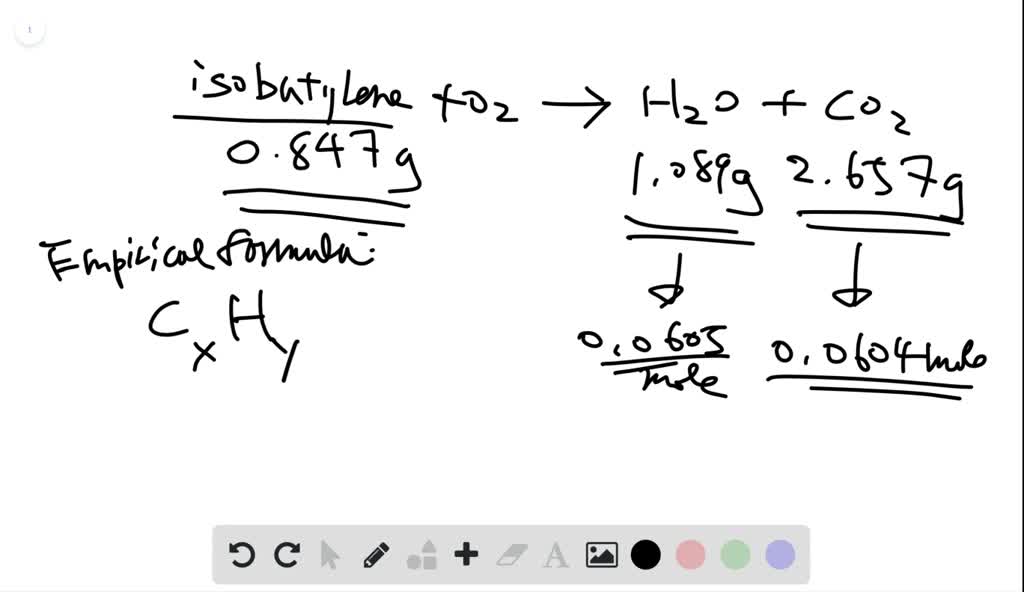
Solved Isobutylene Is A Hydrocarbon Used In The Manufacture Of Synthetic Rubber When 0 847 G Of Isobutylene Was Subjected To Combustion Analysis The Gain In Mass Of The Mathrm Co 2 Absorber Was 2 657 Mathrm G

